orbital diagram for scandium|orbital notation for scandium : iloilo The total number of electrons in scandiumis twenty-one. These electrons are arranged according to specific rules in different . Tingnan ang higit pa LOTTO-Mittel für das Miteinander im Freistaat. Mit LOTTO gewinnt Thüringen – das bedeutet: Von LOTTO Thüringen profitieren nicht nur die Spielerinnen und Spieler mit den richtigen Tippzahlen, sondern auch die Allgemeinheit.. Mehr erfahren
PH0 · scandium valence electrons
PH1 · sc orbital notation
PH2 · orbital notation for scandium
PH3 · orbital diagrams chemistry worksheet
PH4 · orbital diagram for scandium sc
PH5 · obd 2 connector pin chart
PH6 · electron configuration for scandium
PH7 · Iba pa
PH8 · 2 wire sensor wiring
Grand Lotto 6/55 Results Philippine Charity Sweepstakes Office. Saturday, 31 August, 2024. Winning Numbers; 01: 03: 10: 11: 16: 51
orbital diagram for scandium*******Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum number. It is expressed by ‘l’. The . Tingnan ang higit paThe total number of electrons in scandiumis twenty-one. These electrons are arranged according to specific rules in different . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof . Tingnan ang higit paAfter the electron configuration, the last shell of scandium has two electrons and the d-orbital has a total of an electron. Therefore, the valence electrons of scandiumare . Tingnan ang higit paAtoms can jump from one orbital to another orbital in the excited state. This is called quantum jump. The ground state electron configuration of scandium is 1s2 2s2 2p6 . Tingnan ang higit pa
5.8K views 1 year ago. To write the orbital diagram for the Scandium atom (Sc) first we need to write the electron configuration for just Sc. To do that we need to .
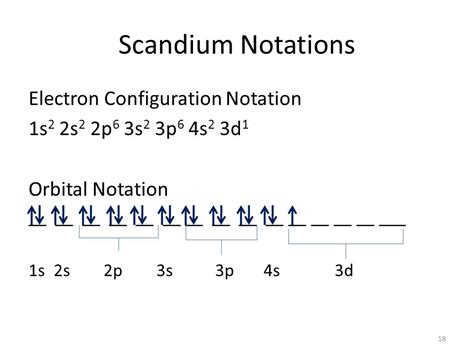
Learn how to write the orbital diagram for scandium (Sc) using the atomic orbitals and the orbital notation. The web page explains the concept of Hund's principle and the sub-energy levels of the s, p, d, . In this video we will see what orbital diagrams are, rules that we follow while determining one for any element and then we will see how to draw one for Sc . Scandium Electron configuration and it's Orbital diagram. Published By Vishal Goyal | Last updated: November 16, 2023. Home » Chemistry » Orbital diagram . In case of Scandium, there are 3 orbits and 17 electrons present in these orbits or shells. Distribution of electrons in shells is 2, 8, and 7. Electron configuration of Sc is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. .
The orbital diagram of scandium shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s .

The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This . Scandium Electron Configuration Diagram. Scandium Electron Configuration Notation. The electron configuration notation for scandium can be .BlockElements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), .
The Bohr Model of Scandium (Sc) has a nucleus that contains 24 neutrons and 21 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The first .Sarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is . The Aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals, but this is actually not true for most elements!From Sc on, the 3d orbitals are actually lower in .orbital notation for scandiumAnswer: The scandium (Sc) electron configuration is written as 1s² 2s²2p6 3s² 3p6 3d¹ 4s². Sub-energy levels are subdivided from atomic energy levels. Orbital levels are sub-energy levels. ‘l’ represents the sub energy levels. The range of values for ‘l’ is 0 to (n – 1). S, p, d, and f are the sub-energy levels.orbital diagram for scandiumScandium (Sc) has an atomic mass of 21. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ChemicalAid. . Orbital Diagram. Nuclear. Radioactive: No: Isotopes. Symbol Mass Number Relative Atomic Mass Isotopic Composition; 36 Sc: 36: 36.01492(54)# 37 Sc: 37: 37.00305(32)# 38 Sc: 38: .
The scandium electron configuration diagram is a valuable tool for understanding the organization of electrons in scandium atoms. By decoding this diagram, we can gain insights into the energy levels and orbital arrangement of scandium’s electrons. To begin decoding the scandium electron configuration diagram, it is important to note that .
Scandium has an atomic number of 21, which means that a scandium atom has a total of 21 electrons. To write the electron configuration of scandium, let’s begin at hydrogen and move across period one of the periodic table, which represents the 1s subshell. . To complete the orbital diagram for the valence shell of titanium, we’ll need to .
Element Scandium (Sc), Group 3, Atomic Number 21, d-block, Mass 44.956. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal .The atomic number of barium is 56, which means it has 56 electrons. Now it is possible to find the orbital notation of barium very easily through electron configuration. That is, the orbital notation of barium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2.
Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We start with a single hydrogen atom (atomic number 1), which consists of one proton and one electron. . Note that for three series of elements, scandium (Sc) through copper (Cu), yttrium (Y .This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their .
Question: Fill in the orbital energy diagram for scandium. 3d 3p E 35 AV AV AV 2p NV 2s AV 1s The lowest Elevels are already filled in for you. Show transcribed image text. There are 2 steps to solve this one.In this case, the scandium atom carries a positive charge. Sc – 3e – → Sc 3+. Here, the electron configuration of scandium ion (Sc 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6. This positive scandium ion (Sc 3+) has twenty-one .orbital diagram for scandium orbital notation for scandiumDraw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)? Express your answer numerically as an integer. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest .The boxes are arranged in order of energy of the orbitals. The lowest energy orbitals are closest to the nucleus and the higher energy orbitals are progressively further away from the nucleus in order of their energy levels. 1. Orbital Diagram for Hydrogen (H) Hydrogen orbital diagram. 2.2022-jul-21 - In this article, we will discuss – Scandium Electron configuration, Orbital diagram, and Valence electrons in detail. Such overlaps continue to occur frequently as we move up the chart. Figure 8.3.1 8.3. 1: Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first.
Where is Housefull 4 streaming? Find out where to watch online amongst 45+ services including Netflix, Hulu, Prime Video.
orbital diagram for scandium|orbital notation for scandium